AI Solutions
Mammography
- Mammography
We have implemented our AI in a Nordic large scale operational screening program, with 80,000 participating woman yearly. They have documented more than 35% workload reduction and better patient outcomes.
Our AI solution has processed more than 4 million mammograms globally and is one of the most mature and well documented AI Software as Medical Device (SaMD).
Several Nordic studies have documents that AI can offer decision support, to detect breast cancer earlier with confidence. Studies provides evidence that detection rate can increase by 20% and up-to 40% of all interval cancers, had been flagged in previous screening round, if AI had been used.
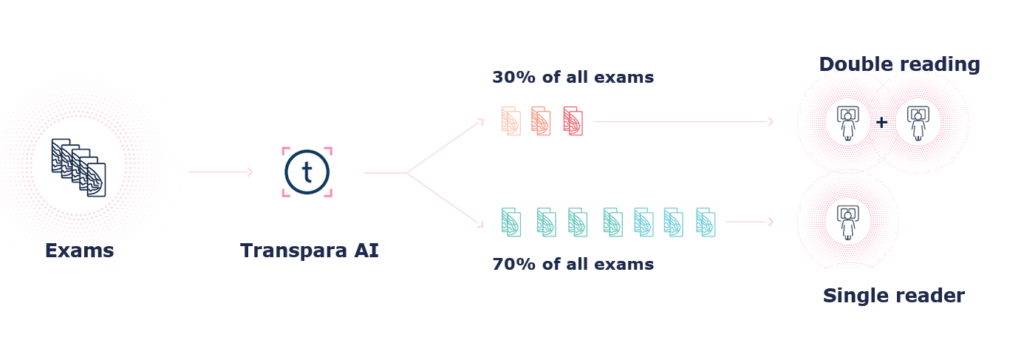
AI can help to risk stratify the participating woman in the screening program. It search across the images for calcifications and soft tissue lesions. The findings are assess for their suspiciousness on a scale from 1-100. Based on the most suspicious findings it gives and overall risk score from 1-10.
Our customers most often decides to categorise 70% of the woman as ‘low-risk’, and then only have single reading. The remaining 30% with ‘elevated risk’ will continue to have double reading. This risk-stratification will reduce the radiologists workload by more than 35% of the total workload in the screening program.
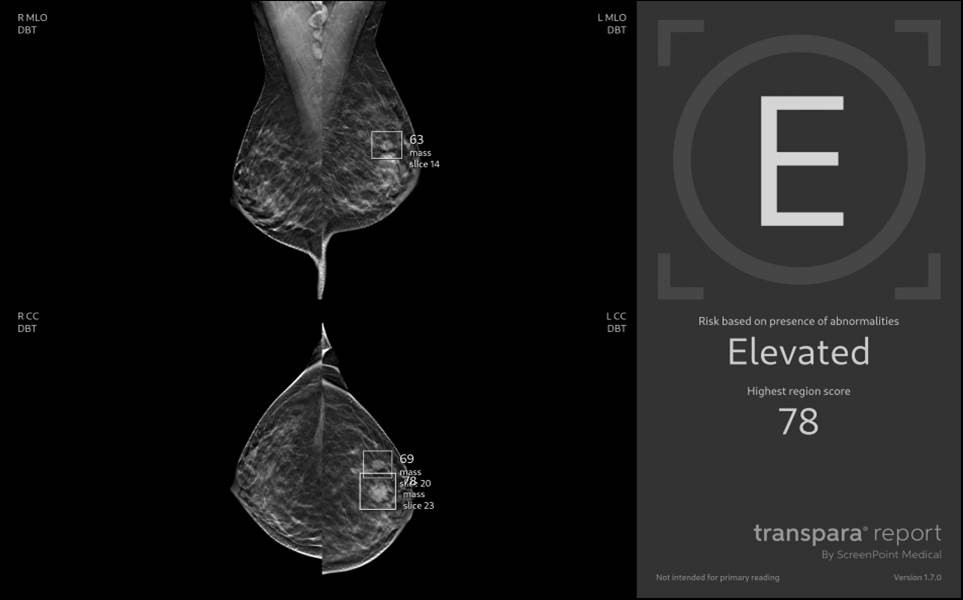
The decision-support give the radiologist more confidence in their decision, especially rule-out and reducing false-positives. This is leading to a significant lower recall rate, saving the woman for anxiety in the waiting time, until they get their final answer in an unpleasant and costly clinical mammography.
Several studies on Nordic populations shows that AI in screening has the opportunity to reduce interval cancer by up-to 40% and increase detection rate with up-to 20%.
Clinical mammography is done for woman who are refered from e.g. the screening program or a general practitioners.
AI offers decision support in the examination to give confidence in the diagnostics, to improve quality and reduce mistakes.
Often tommosynthesis is used in clinical mammography, where the number of images to be examed are significant higher. AI support quick navigation to the most supspicious areas of interest, where it can reduce the work load by up-to 30%.
Our AI can be implemented as a local on-prem installation. all patient data will stay within the hospital network to mitigate any GDPR concerns. Since our AI software is a standard software regulatory approved as a medical device, means no local adoption is required. It works with standard data formats and is 100% vendor agnostic, hence integrates with all PACS and RIS systems.
Screepoint Medical is working with breast clinicians to continually improve the clinically proven deep learning algorithms in Transpara®. We are committed to further improve accuracy, consistency and reduce unnecessary recalls, a common cause of anxiety for women undergoing mammography.
ScreenPoint Medical was founded in 2014 by Professor Nico Karssemeijer and Professor Sir Michael Brady, world leading experts in quantitative breast image analysis and computer aided detection.
The resulting product, Transpara, has featured in multiple peer-reviewed publications that show it enables significant clinical improvements to the timely diagnosis of breast cancer.